A RELIABLE TECHNIQUE FOR MINOR SALIVARY GLAND BIOPSY: A SAFE AND EFFECTIVE METHOD FOR DIAGNOSING SJÖGREN'S SYNDROME IN SERONEGATIVE PATIENTS
Summary
Aims: To describe a safe and standardized technique for performing minor salivary gland biopsy and to highlight its diagnostic value in patients with seronegative Sjögren's syndrome.Material and Methods: Medical records of 319 patients who presented to the Rheumatology Clinic at Izmir Bakircay University Faculty of Medicine Cigli Training and Research Hospital between January 2020 and July 2024 were retrospectively reviewed. These patients had undergone serological testing for suspected SS and were subsequently referred to the ENT Department for minor salivary gland biopsy.
Results: Among the patients, 43 (13.4%) had negative serological results but exhibited MSGB scores of 3 or higher. SS was directly diagnosed in 92 patients (28.8%) based on Chisholm-Mason scores of 3 or 4. Additionally, some of the 167 patients (52.3%) with scores of 1 or 2 were diagnosed with SS based on supplementary clinical findings, leading to the initiation of treatment. MSGB findings contributed to the diagnosis in more than half of the suspected SS cases. Follow-up examinations one month post-biopsy revealed that most patients did not experience adverse symptoms such as pain, numbness, burning, tingling, or swelling.
Conclusion: Our study underscores the critical role of MSGB in diagnosing Sjögren's syndrome, particularly in seronegative patients. Given its safety and importance, MSGB remains a reliable and essential method for identifying SS.
Introduction
Sjögren syndrome (SS) is an autoimmune disease characterized by inflammation of the exocrine glands, predominantly causing xerostomia and/or xerophthalmia and, less commonly, systemic symptoms. In the United States, an average of 5.8 out of 100,000 people are diagnosed with SS each year, the majority of whom are women. When the disease is suspected, definitive diagnosis is based on a combination of serologic evidence, oral and ocular secretion studies, and pathologic findings[1,2]. In addition to the lacrimal and salivary glands, the disease can also affect other exocrine glands: the lungs, kidneys, and blood vessels. The presence of the disease alone is defined as primary SS (pSS), while the presence of another autoimmune disease is defined as secondary SS (sSS)[3].SS is diagnosed based on the criteria accepted by the American-European Consensus Group in 2002. These are the presence of ocular and oral dryness combined with objective evidence of autoimmunity with a positive minor salivary gland biopsy (>1 focus count), positive anti-Sjögren syndrome-associated antigen A (anti SS-A/Ro) or anti-Sjögren syndrome-associated antigen B (anti SS-B/La) antibodies, and the presence of keratoconjunctivitis sicca with a positive Schirmer test with <5 mm wetting of the filter paper in 5 minutes[4,5].
Since the diagnosis of SS is challenging, multiple diagnostic tools are typically required. Minor salivary gland biopsy (MSGB) plays a crucial role, particularly in diagnosing seronegative SS patients[6,7]. The MSGB procedure is relatively simple and can be performed with local anesthesia on an outpatient basis[8]. The most common complication after a minor salivary gland biopsy is numbness. In addition, complications such as minor bleeding, hematoma, local infection, premature cessation of sutures, keloid, and granuloma formation may also occur[9]. The MSGB is undoubtedly important for the classification, diagnosis, and prognosis of SS. Three main classification systems, proposed by Chisholm and Mason, Greenspan and Daniels, and Tarpley, are still widely used for interpreting biopsy results[6]. In their original study, Derrick Chisholm and David Mason obtained MSGB samples from 40 patients and 60 cadavers. According to this study, the classification of Chisholm and Mason, defined in 1968, uses a 5-grade classification from 0 to 4 based on the presence of mild or moderate lymphocytic infiltration and/or lymphocytic foci.
The Chisholm and Mason scoring system evaluates the degree of lymphocytic infiltration per 4 mm² of salivary tissue as follows:
• Grade 0: No infiltration
• Grade 1: Mild infiltration
• Grade 2: Moderate infiltration or multiple foci
• Grade 3: A single focus
• Grade 4: Multiple foci
A focus is defined as an accumulation of 50 or more lymphocytes per 4 mm². Chisholm and Mason observed that all patients with salivary duct antibodies had a positive biopsy (grade 3 or 4). They concluded that MSGB could serve as a valuable diagnostic tool for Sjögren's syndrome[10]. Since then, this grading system has been widely used by pathologists; it had a sensitivity of 72.1% and a specificity of 80% in a study for SS, respectively[11].
In light of this information, this study aims to describe a safe and standardized technique for performing minor salivary gland biopsy and to highlight its diagnostic value in patients with seronegative Sjögren's syndrome.
Methods
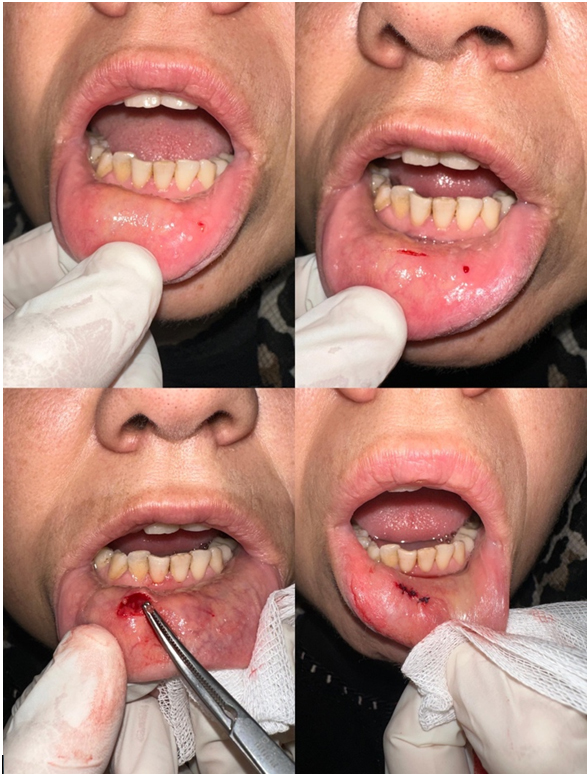
The medical records of 319 patients who went to the Rheumatology Clinic at the Izmir Bakircay University Hospital, had serological tests that led to a preliminary diagnosis of SS, and were then sent to the ENT Department for a minor salivary gland biopsy between January 2020 and July 2024 were looked at retrospectively. This study was approved by the local clinical research ethics committee in Izmir Bakircay University. This study was conducted according to the World Medical Association Declaration of Helsinki and all the patients signed informed consent forms for inclusion to the study.From the blood tests, the anti-nuclear antibody (ANA), anti-Sjögren syndrome-associated antigen A (Anti SSA/Ro), anti-Sjögren syndrome-associated antigen B (Anti-SSB), and rheumatoid factor (RF) values were tracked. We recorded the results of the patients who underwent ophthalmology consultation and the Schirmer test. Under local anesthesia, the same technique and procedure were used on all patients for a minor salivary gland biopsy. An incision was made about 3 cm from the lower lip mucosal surface to the vermillion line, as shown in Figure 1 (Lower lip minor salivary gland biopsy procedure). The procedure involved stretching the lower lip outward. Apply 0.5-1 ml of 1% lidocaine and 1/100000 adrenaline to the submucosa. Then, a superficial incision of approximately 2 cm parallel to the vermillion border is made with a number 15 scalpel. We use blunt dissection to separate the minor salivary glands from their fascia, working parallel to the sensitive nerves. After at least 4 minor salivary gland tissues are removed, the incision is closed by primary suturing with 4/0 Vicryl. Tissue samples were placed in formalin and forwarded to pathology, where a single experienced head and neck pathologist examined all specimens to ensure diagnostic consistency. A pathologist with expertise in Sjögren's syndrome evaluated the biopsy specimens, determined the number of lymphocytic foci, and scored them using the Chisholm-Mason scoring system.
 Büyütmek İçin Tıklayın |
Figure 1: Lower lip minor salivary gland biopsy procedure |
All patients' epicrisis reports at the 1st month follow-up were reviewed, and their answers to questions about possible complications asked to minor salivary gland patients in our clinic were examined. The patients' demographic, histopathological, and serological features were looked at. The results were also looked at, along with the percentage rates that showed how often the findings happened.
Statistical Analyses
Only descriptive statistics were reported; no hypothesis testing, confidence intervals, or modeling were performed. Categorical variables were presented as counts (percentages).
Histopathology was tabulated by Chisholm?Mason grades and (if available) focus score; serologic results (ANA, anti-SSA/Ro, anti-SSB/La, RF) and Schirmer testing were summarized as n (%). Procedure-related symptoms/complications at 1 month were reported as prevalence. No imputation was performed for missing data; the number of evaluable cases (denominator) for each variable is indicated in the tables/text. No subgroup or between-group comparisons were conducted. Summaries were generated using the IBM SPSS Statistics version 25.0 software (IBM Corp., Armonk, NY, USA).
Results
A total of 319 MSGB with a preliminary diagnosis of SS were included in the study. Of these patients, 288 (90.3%) were female and 31 (9.7%) were male. Serological evaluation revealed that anti-nuclear antibody (ANA) was negative in 224 patients (70.2%) and positive in 90 patients (28.2%), while ANA results were unavailable for 5 patients. Anti-SSA (Ro) was negative in 236 patients (73.9%) and positive in 10 patients (3.1%), with 73 patients (22.9%) having missing data. Similarly, anti-SSB (La) was negative in 242 patients (75.8%) and positive in 4 patients (1.2%), and the results were not available for 73 patients. Rheumatoid factor (RF) was below 10 in 234 patients (73.3%), between 10?14 in 21 patients (6.5%), and above 14 (considered positive) in 43 patients (13.4%); RF data were missing for 21 patients.Ophthalmologic examination using the Schirmer test showed values of ≤5 mm in 83 patients (26%), 6-14 mm in 37 patients (11.5%), and ≥15 mm in 6 patients (1.8%), while the test was not performed or the data were unavailable in 203 patients (63.6%).
The Chisholm-Mason scores obtained from MSGB are summarized in Table 1. A definitive diagnosis of Sjögren's syndrome was made in 92 patients (28.8%) with Chisholm-Mason scores of 3 or 4. Additionally, 43 patients (13.4%) were diagnosed with SS based on MSGB scores of 3 or higher, despite having negative serological results. Among the 167 patients (52.3%) with scores of 1 or 2, some were diagnosed with SS based on additional clinical findings, and treatment was initiated accordingly. In contrast, a diagnosis of SS was excluded in 60 patients (18.8%) who had a score of 0.
Table 1: Chisholm-Mason scores and importance of MSGB
Postoperative follow-up data were available for 282 patients who returned for their one-month evaluation. Among these, 241 patients (85%) reported no pain, and only 10 patients (3.5%) experienced pain. Swelling was reported by 11 patients (4%), while 250 patients (88%) had no swelling. Burning or tingling sensations were noted by 15 patients (5%), whereas 251 patients (89%) reported no such complaints. Numbness was reported by 9 patients (3%), and 253 patients (90%) reported no numbness. Importantly, no hematomas were observed in any of the patients.
Discussion
Sjögren's syndrome is a systemic autoimmune disease characterized by chronic plasmacytic infiltration of all exocrine glands, particularly the salivary and lacrimal glands[12]. The diagnosis of SS is challenging due to the lack of highly specific clinical and paraclinical parameters. Therefore, through our study, we aimed to emphasize the importance of this procedure, which is commonly performed in ENT practice but whose clinical value remains underappreciated, and to highlight its relevance in routine clinical decision-making.Since 1965, various diagnostic criteria have been proposed. The European Classification Criteria were introduced in 1993 and later revised by the American-European Consensus Group (AECG) in 2002. The AECG aimed to enhance diagnostic specificity by requiring evidence of autoimmunity, either through the presence of anti-Ro or anti-La autoantibodies or focal lymphocytic sialadenitis on MSGB(5). As a result of extensive research, the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR) established the EULAR/ACR classification criteria in 2016. These criteria have since become a crucial tool for evaluating and diagnosing Sjögren's syndrome (Table 2) [13]. A distinguishing feature of SS is B-cell hyperreactivity, which leads to the production of autoantibodies such as ANA, including anti-Ro60, anti-Ro52, and anti-La, as well as rheumatoid factors (RF) [14]. These autoantibodies are present in only 45% to 75% of patients with Sjögren's syndrome[15]. In patients lacking serum anti-Ro52/Ro60 (SSA/Ro) antibodies, the diagnosis of SS is based on internationally established classification criteria. These include reduced tear and/or salivary flow and the presence of focal lymphocytic sialadenitis on MSGB[13]. Patients who meet the diagnostic criteria for SS but do not test positive for conventional serum autoantibodies are classified as seronegative SS. However, there is no universally accepted definition of which autoantibodies should distinguish seropositive SS from seronegative SS. Consequently, the reported prevalence of seronegative SS in the literature ranges from 8% to 37%.
Table 2: The 2016 ACR/EULAR Classification Criteria for pSS
Due to the absence of specific autoantibody biomarkers, seronegative SS may be underdiagnosed in clinical settings unless further diagnostic evaluations, such as MSGB, are performed[16].
In our study, despite negative serological results in 43 (13.4%) patients, MSGB scores of 3 or higher were observed. SS was directly diagnosed in 92 (28.8%) patients with Chisholm-Mason scores of 3 and 4. Additionally, some of the 167 (52.3%) patients with scores of 1 and 2 were diagnosed with SS based on additional clinical findings, leading to the initiation of treatment. In more than half of the patients suspected of having SS, the diagnosis was established using MSGB findings. These results highlight the critical role of MSGB, particularly in seronegative SS patients.
Follow-up epicrisis evaluations at one month post-biopsy revealed that the majority of patients did not report symptoms such as pain, numbness, burning/tingling, or swelling. We believe this outcome is associated with the superficial biopsy technique employed in our clinic. Our method involves stretching and everting the lower lip, which enhances the visibility of minor salivary glands. Additionally, local anesthetic infiltration into the submucosa facilitates separation of the epithelium from the glands. Beginning the procedure with a very superficial incision using a scalpel, without damaging underlying structures, is another key aspect. Furthermore, performing glandular dissection parallel to nerve fibers may prevent the development of numbness or sensory loss.
Consistent with our findings, Kim et al. [17] reported similar outcomes using a comparable technique; by contrast, deeper biopsy approaches have been associated with higher complication rates[18].
Complications associated with minor salivary gland biopsy (MSGB) are uncommon. Reported rates include immediate bleeding in approximately 7?8% of cases and hematoma in 2?3%. Persistent sensory disturbance (numbness or paresthesia) is infrequent, typically 1?6% across published series[19]. Consistent with these data, we observed minimal complications in our cohort; we attribute this to maintaining a superficial dissection plane parallel to the course of the sensory nerve fibers.
Based on our findings, we propose that numbness, the most frequently reported post-biopsy complication in the literature, is primarily due to deeper biopsy techniques. We believe that our method minimizes complication rates, but further prospective, well-controlled studies are necessary to obtain more objective data regarding this technique.
In recent years, research has also explored the molecular basis of SS, an autoimmune disease. Salivary gland biopsy materials have been analyzed for various parameters, including DNA microarray profiles, monocyte chemotactic protein-1 receptor presence, IL-22-producing cell levels, expression of IL-17, IL-23, and their respective receptors.
Another indispensable aspect of salivary gland biopsy is its role in assessing lymphoma risk. Patients with SS have an increased risk of lymphoma compared to the general population, making MSGB crucial not only for routine histopathological evaluation but also for detecting ectopic germinal centers, a key indicator of lymphoma development[20]. Additionally, Jonsson et al. reported that the presence or absence of germinal center-like structures in biopsy samples from patients diagnosed with pSS can be used to classify patient subgroups based on serological profiles, thereby aiding in prognostic prediction[21]. Beyond focus score, histologic/immunohistochemical features such as ectopic germinal center-like structures (identified with markers including BCL6/CD20/CD21) carry prognostic significance?including association with B-cell activation and lymphoma risk?and may refine risk stratification in future cohorts. Circulating and tissue biomarkers (e.g., CXCL13 as a surrogate of lymphoid organization; salivary/serologic panels reflecting B-cell activation) are promising for monitoring disease activity and could be integrated with LSGB readouts in prospective studies[22]. These findings highlight just a few of the benefits that MSGB offers in the differential diagnosis of Sjögren's syndrome.
Limitations
The most significant limitation of our study is its retrospective design, which resulted in the inaccessibility of some data. Based on our findings, it is evident that more objective and robust conclusions could be achieved through prospective studies involving larger patient cohorts, specifically designed to calculate the sensitivity, specificity, and positive predictive value (PPV) of MSGB, thereby more accurately determining its diagnostic utility.
Conclusion
Minor salivary gland biopsy is undeniably significant in diagnosing Sjogren's syndrome. Our study highlights the significance of minor salivary biopsy in diagnosing Sjogren's disease, particularly in seronegative patients. MSGB, utilizing the approach we employed, is a secure and significant way to diagnose Sjogren's syndrome.We aimed to underscore the significance of this evaluation, commonly conducted in ENT practice, whose usefulness we believe remains inadequately recognized, through our study. We believe that minor salivary gland biopsy will assume an increasingly significant role in the diagnosis and treatment of the disease due to advancements in immunohistochemistry techniques and biomarkers.
Reference
1) Gordon AJ, Patel A, Zhou F, Liu C, Saxena A, Rackoff P, et al. Minor salivary gland biopsy in diagnosis of Sjögren's syndrome. OTO open. 2022;6(3):2473974X221116107.
2) Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, Bosch X. Primary Sjögren syndrome. Bmj. 2012;344.
3) YILMAZ MS, GÜVEN M, AKIDİL Ö, YAZICI A, KAYABAŞOĞLU G, KAHYAOĞLU Z. SJÖGREN SENDROMU AYIRICI TANISINDA ALT DUDAK MİNÖR TÜKRÜK BEZİ BİYOPSİSİNİN TANISAL DEĞERİ. KBB-Forum 2013;12(3)
4) Qin B, Wang J, Yang Z, Yang M, Ma N, Huang F, et al. Epidemiology of primary Sjögren's syndrome: a systematic review and meta-analysis. Annals of the rheumatic diseases. 2015;74(11):1983-9.
5) Vitali C. Classification criteria for Sjögren's syndrome. Annals of the rheumatic diseases. 2003;62(1):94-5.
6) Bautista-Vargas M, Vivas AJ, Tobón GJ. Minor salivary gland biopsy: Its role in the classification and prognosis of Sjögren's syndrome. Autoimmunity reviews. 2020;19(12):102690.
7) Caporali R, Bonacci E, Epis O, Bobbio?Pallavicini F, Morbini P, Montecucco C. Safety and usefulness of minor salivary gland biopsy: retrospective analysis of 502 procedures performed at a single center. Arthritis Care & Research: Official Journal of the American College of Rheumatology. 2008;59(5):714-20.
8) Wicheta S, Van der Groen T, Faquin WC, August M. Minor salivary gland biopsy?an important contributor to the diagnosis of Sjögren syndrome. Journal of Oral and Maxillofacial Surgery. 2017;75(12):2573-8.
9) Colella G, Cannavale R, Vicidomini A, Itro A. Salivary gland biopsy: a comprehensive review of techniques and related complications. Rheumatology. 2010;49(11):2117-21.
10) Chisholm D, Mason D. Labial salivary gland biopsy in Sjögren's disease. Journal of clinical pathology. 1968;21(5):656-60.
11) Cui G, Sugai S, Ogawa Y, Takeshita S, Masaki Y. Evaluation of the histologic criteria for the diagnosis of Sjögren's syndrome. Japanese Journal of Clinical Immunology. 1999;22(2):72-9.
12) Feltsan T, Stanko P, Mracna J. Sjögrens syndrome in present. Bratisl lek listy. 2012;113(8):514-6.
13) Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Annals of the rheumatic diseases. 2017;76(1):9-16.
14) Lee AY, Wang JJ, Gordon TP, Reed JH. Phases and Natural History of Sjögren's Disease: A New Model for an Old Disease? Arthritis Care & Research. 2023;75(7):1580-7.
15) Retamozo S, Acar-Denizli N, Horváth IF, Ng W-F, Rasmussen A, Dong X, et al. Influence of the age at diagnosis in the disease expression of primary Sjögren syndrome. Analysis of 12,753 patients from the Sjögren Big Data Consortium. Clinical and experimental rheumatology. 2021;39(6):S166-S74.
16) Lee AY, Rischmueller M, Reed JH. Seronegative sjögren syndrome: a forgotten entity? : The Journal of Rheumatology; 2024. p. 7-9.
17) Kim J, Sun D, Ozl R, Grader?beck T, Birnbaum J, Akpek EK, et al. A validated method of labial minor salivary gland biopsy for the diagnosis of Sjögren's syndrome. The Laryngoscope. 2016;126(9):2041-6.
18) Pellegrini M, Pulicari F, Zampetti P, Scribante A, Spadari F, editors. Current salivary glands biopsy techniques: a comprehensive review. Healthcare; 2022: MDPI.
19) Santiago ML, Seisdedos MR, Salinas RNG, Secco A, Claverie LM, Techera L, et al. Frequency of complications and usefulness of the minor salivary gland biopsy. Reumatología Clínica (English Edition). 2012;8(5):255-8.
20) Vitali C, Minniti A, Pignataro F, Maglione W, Del Papa N. Management of Sjögren's syndrome: present issues and future perspectives. Frontiers in Medicine. 2021;8:676885.
21) Jonsson MV, Skarstein K. Follicular dendritic cells confirm lymphoid organization in the minor salivary glands of primary Sjögren's syndrome. Journal of oral pathology & medicine. 2008;37(9):515-21 [ Özet ]