LANGUAGE DEVELOPMENT IN CHILDREN WITH UNILATERAL HEARING LOSS: UNILATERAL AURAL ATRESIA AND SINGLE SIDED DEAFNESS
2Hacettepe Üniversitesi Sağlık Bilimleri Fakültesi, Odyoloji Bölümü, Ankara, Türkiye
Summary
Objective: The aim of this study is to investigate the effects of single sided deafness (SSD) and unilateral aural atresia (UAA) on receptive and expressive language skills, as well as to compare these skills in children with SSD, UAA, and NH.Material and Methods: This study included 12 children with SSD, 15 children with UAA, and 15 children with normal hearing (NH). Their ages ranged from 2 to 8 years. The Turkish-Early Language Development Test (TEDIL) was used to evaluate the receptive and expressive language development of the children.
Results: The statistical analysis revealed that there was no significant difference between the SSD and UAA groups' receptive, expressive, and spoken language skills (p>0.017), whereas the NH group had significantly higher receptive, expressive, and spoken language skills than both groups (p<0.017).
Conclusion: The lack of differences between the SSD and UHL groups and the poorer language skills compared to those with NH suggest that children with UAA and SSD appear to have significant risk for receptive and expressive language delays. In order to reduce the possibility that these children will lag behind their peers in receptive and expressive language, it is crucial that their language development should be evaluated carefully. Also, further studies are needed to determine what kinds of auditory amplification or special education are effective in rehabilitating children with UHL for their delayed language development.
Introduction
Unilateral hearing loss (UHL) is defined as normal hearing in one ear and permanent hearing loss of any degree and configuration in the other ear[1]. The prevalence of UHL is estimated at 1 per 1000 children at birth[2]. Children with UHL may have sensorineural hearing loss (SNHL), which is caused by a defect in the inner ear (cochlea) and/or eight cranial nerve. Single-sided deafness (SSD) is the term used to describe a severe-to-profound sensorineural hearing loss in one ear and normal hearing in the other[3]. UHL can also frequently caused by conductive pathologies such as chronic otitis media, congenital ossicular malformation and aural atresia[2]. Aural atresia is congenital difference characterized by an underdevelopment or total absence of the external auditory canal associated with variable middle ear effects. In unilateral aural atresia (UAA), the atresia of the ear canal typically leads to conductive hearing loss while the contralateral ear is unaffected[4].In the past, it was considered that UHL has minimal effects on the speech and language development of children[5]. Currently, delay in many aspects has been documented in children with UHL[1,5]. Since binaural hearing demonstrates the advantages of head shadow effect, binaural unmasking, and binaural summation, these effects would reduce the hearing burden and enable children to concentrate more on speech in the target ear[6]. Children with monaural hearing would pay more attention to localize sounds and discriminate them from background noise if their binaural hearing was impaired, delaying the development of their auditory, speech, and language skills[6]. As a result, it is expected that children with UHL will have a tendency to lag behind compared to their peers with NH in terms of their auditory and language abilities. However, it is unclear whether the type of HL in the ear with hearing impairment will have a similar impact on these abilities. Because in the majority of research, the auditory and language skills of a group with a certain type of UHL are compared to those of their NH peers. Therefore, the aim of this study is to investigate the effects of single sided deafness (SSD) and unilateral aural atresia (UAA) on receptive and expressive language skills, as well as to compare these skills in children with SSD, UAA, and NH
Methods
This study was conducted at Hacettepe University Faculty of Health Sciences, Audiology Department. It was performed in line with the principles of the Declaration of Helsinki and received ethical approval from the Hacettepe University Health Science Research Ethics Board (G023/655). The participants and their parents provided informed consent on the day of enrollment.
1. Participants
This study involved 42 children between the ages of 2 and 8, including children with SSD, UAA, and NH to assess the impact of different types of UHL on language development during childhood. The characteristics of the sample are summarized in Table 1.
Table 1: Demographics of the participants
In this study, the participants are patients who have been appointed to our department for auditory rehabilitation evaluation following the completion of hearing assessments. In order to participate in this study, children with UAA and SSD were required to meet all of the following criteria: 1) passed the newborn hearing screening contralateral side, 2) not fitted with amplification or hearing aided device, 3) had normal outer and middle ear function contralateral side. 4) for children with SSD; having profound unilateral HL prelingually according to the classification of degree of HL made by the American-Speech-Language-Hearing Association (ASHA)[7], and having a normal hearing in the contralateral ear (in ABR test; at click stimulus, air conduction threshold ≤ 20 dB, or in behavioral audiometry, pure tone average (PTA) of 500, 1000, and 2000 Hz ≤ 15 dB), 5) for children with UAA having normal bone conduction threshold in the atretic ear (in ABR test; at click stimulus bone conduction threshold ≤ 20 dB nHL, or in behavioral audiometry having threshold at 500, 1000, 2000 and 4000 Hz ≤ 15 dB HL), and having a normal hearing in the contralateral ear ear (in ABR test; at click stimulus, air conduction threshold ≤ 20 dB, in behavioral audiometry, pure tone average (PTA) of 500, 1000, and 2000 Hz ≤ 15 dB). Children diagnosed with syndromic hearing loss, neurological or developmental disorders, learning difficulties, and other comorbidities were excluded in this study.
The SSD group consisted of 12 children (7 females and 5 males); their ages ranged from 2.01 to 8 years (mean age ± SD: 5.20 ± .59 years). Two of the children's mothers graduated from elementary school, seven graduated from high school, and three had a bachelor's degree. In addition, 7 children with SSD had normal inner ear and cochlear nerve anatomy, and the etiology of their HL was unknown, whereas 5 of them had cochlear nerve (CN) aplasia.
The UAA group consisted of 15 children (4 females, 11 males), ages 2.08 to 6.32 years (mean age ± SD: 4.11 ± 0.35 years). Five of the children's mothers graduated from elementary school, 7 graduated from high school, and 3 had a bachelor's degree.
The NH group included 15 children (10 females, 5 males), ages 2.01 to 7.80 years (mean age ± SD: 4.58 ± .45 years ). They had PTA≤15 dB HL bilaterally. Also, they passed newborn hearing screening for both ears. Three of the children's mothers graduated from elementary school, 6 graduated from high school, and 6 had a bachelor's degree.
2. Test Battery
Participants" receptive and expressive language development was measured by the Turkish-Early Language Development Test (TEDIL), a Turkish adaptation of the Test of Early Language Development (TELD-3) developed by Hresko, Reid, and Hammill (1999)[8]. The normative data for the TEDIL consisted of 1200 normally developing children aged between 18 months and 8 years. The validity and reliability results are strong and significant. The results showed that TEDIL accurately examines receptive and expressive language, and identified children with language delay and language disorders[9].
The test consists of verbal instructions given to the child, with stimuli as objects or pictures, to which the child is asked to response. In this study, we presented TEDIL scores as standard scores. The receptive and expressive subtests scores are combined to determine the spoken language standard score. Scoring ranges from 35 to 165. The scoring system was presented in Table 2.
3. Statistical Analysis
The G* Power program was used to determine the sample size to be included in the study. Considering the mean and standard deviation values obtained from the groups as a result of pilot study, this study should include 6 participants from each group with a 5% type I error level and 95% power to detect a minimal, clinically significant difference.
SPSS version 23 was used to analyze data. The variables' normality was determined using histograms, probability plots, and Kolmogorov-Smirnov/Shapiro-Wilk's test. Means, standard deviations, and percentages were used for descriptive analysis. The Kruskal-Wallis test was performed for multiple comparisons, and the Mann-Whitney U test was used to examine the significance of pairwise differences with Bonferroni correction. Chi-Squared analysis compared categorical variables. p<0.05 indicated statistical significance.
Results
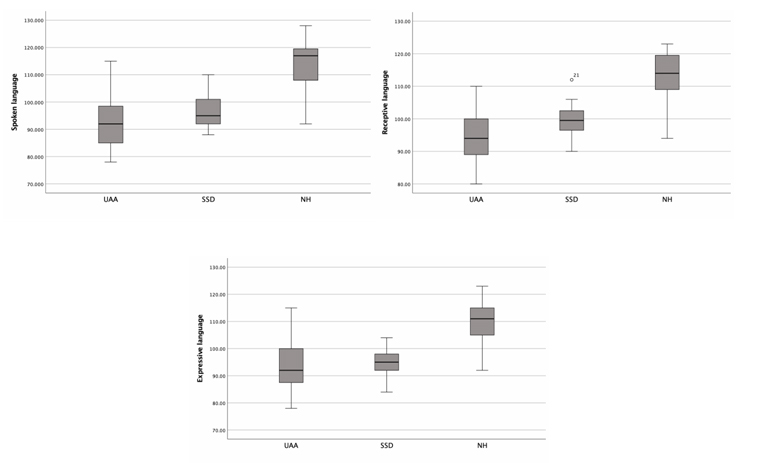
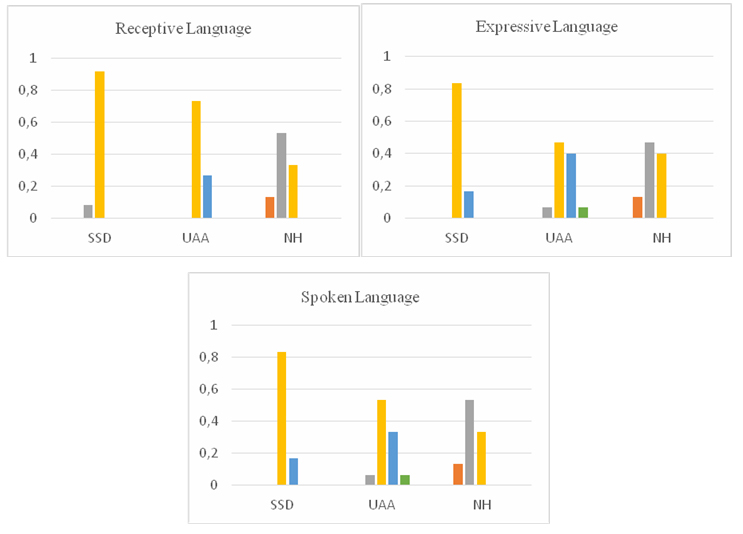
The mean receptive language standard score 99.66 ± 5.98 (min: 90, max: 112) for the SSD group, 94.46 ± 8.21 (min: 80, max: 110) for the UAA group, and 111.80 ± 9.40 (min: 94, max: 123) for the NH group.When the levels of receptive language were examined, 8.3% (n=1) of the SSD group were above average, while 91.7% (n=12) were at the average level. In the UAA group, 26.7 % (n=4) of the participants were below average, while 73.3 % (n=11) were average. In the NH group, 13.3 % (n=2) of the participants were good, 53.3 % (n=8) were above average, and 33.3 % (n=5) were at the average level.
The mean expressive language standard score 94.41 ± 5.80 (min: 84, max: 104) for the SSD group, 93.6 ± 9.47 (min: 78, max: 115) for the UAA group, and 109.6 ± 9.44 (min: 92, max: 123) for the NH group.
When the levels of expressive language were examined, 16.7 % (n=2) of the SSD group were below average, while 83.3 % (n=10) were at the average level. In the UAA group, 6.7 % (n=1) of the participants were poor, 40 % (n=6) were below average, 46.7 % (n=7) were average, and 6.7% (n=1) were at the above average level. In the NH group, 13.3 % (n=2) of the participants were good, 46.7 % (n=7) were above average, and 40% (n=6) were at the average level.
The mean spoken language standard score 96.5 ± 6.25 (min: 88, max: 110) for the SSD group, 92.86 ± 10.02 (min: 78, max: 115) for the UAA group, and 112.93 ± 11.1 (min: 92, max: 128) for the NH group.
When the levels of spoken language language were examined, 16.7 % (n=2) of the SSD group were below average, while 83.3 % (n=10) were at the average level. In the UAA group, 6.7 % (n=1) of the participants were poor, 33.3 % (n=5) were below average, 53.3 % (n=8) were average, and 6.7% (n=1) were at the above average level. In the NH group, 13.3 % (n=2) of the participants were good, 53.3 % (n=8) were above average, and 33.3 % (n=5) were at the average level.
Comparing the standard scores for receptive, expressive, and spoken language revealed statistically significant differences between the groups (p<0.001). Post-hoc analysis showed that SSD and UAA group had similar scores, but NH had a significantly higher scores (p<0.017) (Fig. 1, Table 3)
 Büyütmek İçin Tıklayın |
Fig 1: Comparison of TEDIL scores between groups |
Table 3: The Kruskal-Wallis Analysis of TEDIL assesment and post-hoc results
In addition, comparison of receptive, expressive, and spoken language level showed significant differences between the groups (p<0.001). In the post-hoc analysis, the levels of the SSD and UAA groups were not significantly different, however the levels of NH were different (p<0.017) (Fig. 2, Table 3).
 Büyütmek İçin Tıklayın |
Fig 2: Comparison of TEDIL levels between groups |
Discussion
This study compared the language skills of children with SSD, UAA, and NH. Although numerous studies have shown that UHL has a variety of adverse outcomes in children, there are also a few conflicting findings. According to some previous studies, there was no difference in language skills between children with UHL and their NH pers[10,11]. On the other hand, more recent studies suggest that UHL can be detrimental to phonological processing, word recognition, vocabulary acquisition, and language development[12,13]. In these recent studies, a comparison was made between two groups: children with varying degrees and types of UHL and their NH peers. It was concluded that children with UHL were at risk for language development, and that when binaural auditory function is impaired, children with normal monaural hearing will focus more on auditory perception skills (sound detection, discrimination, and identification). Development of speech and language is closely related to auditory skills. Therefore, abnormal auditory skills will interfere with the acquisition of meaningful speech, resulting in poor language skills[6].In this study, unlike previous studies, we included two UHL groups with different HL types. Because we actually aimed to evaluate the effects of different types of UHL, such as SSD and UHL, on language skills.
Firstly, we compared the receptive, expressive and spoken language scores between the groups. We found that there was no significant difference between the SSD and UAA groups, whereas NH group had higher receptive, expressive, and spoken language scores than the both groups. In a case-control study, Sangen et al. (2017) compared the language and auditory development of children with congenital SSD to that of NH children. They indicated that children with SSD had lower scores on tests of morphology, syntax, and vocabulary, presumably due to disrupted auditory input during the time of normal language acquisition[14]. Takeyama et. al. (2022) examined the whether pre-school age children with SSD have delays in the development of receptive vocabulary and verbal intelligence. They suggested that the development of receptive vocabulary and verbal intelligence was delayed in pre-school age children with SSD[5]. Lieu and colleagues' research has also given insight on language difficulties in children with various degrees and types of UHL. They showed that children with UHL scored significantly lower on receptive and expressive language tests than their NH pers[15]. They also found that while oral language scores for children with UHL improved significantly over time, their receptive and expressive language ability remained worse than those of their NH pers[16]. While these findings are significant, it is unclear if they can be extrapolated to children with UAA because relatively few studies have focused on language difficulties in children with UAA. In their study, Montino et al. (2017) compared the language development of children with unilateral and bilateral AA and found no significant differences between their language skills. In addition, they reported that children with AA had poorer speech and language skills than their NH pers[17]. Jensen et al. (2013) investigated the whether increased risk for speech and learning problems exists among children with AA, and they found that children with UAA may have a similar risk of speech and learning difficulties as children with unilateral sensorineural hearing loss[18]. In accordance with the findings of the literature and this study, Wieringen et al. (2019) stated that language acquisiton requires the integration of perceptual information, and that even mild hearing loss can impede this process[19]. Considering these findings, it was expected that children with NH would have superior language skills than children with UHL. However, there was no difference between SSD and UAA groups indicating that various types of UHL may have similar effects on language skills.
Second, we examined the groups" levels of receptive, expressive, and spoken language. We found that the levels of receptive, expressive, and spoken language of children with UAA and SSD were mainly average. At first glance, these results suggest that children with UHL achieve average scores on standardized test normative measures. However, as Tomblin et al. (2015) point out, it may be more meaningful to compare outcomes with NH peers from the same population, as standardized test scores could underestimate their actual potential[20]. Fitzpatrick et. al. (2011) reported that while children with mild to profound HL scored within test norms, they lagged significantly behind their NH peers based on a control group[21]. Similarly, in the current study, receptive, expressive, and spoken language levels of children with UAA and SSD were within the test norms, but they were actually behind their NH peers from the same age population.
The effects of congenital UHL on language development explained by the maturation of central auditory system. Although the cochlea reaches maturation by week 23 of gestation, the emergence of binaural hearing ability and the subsequent development of auditory processing and perception require over a decade. This is the only way redundancy, head shadow, squelch, and cocktail party effects can help with sound localization, speech recognition in background noise, and spatial hearing. Therefore, individuals diagnosed with UHL encounter challenges in accurately localizing sounds and separating speech signals from background noise. Inadequate reception of acoustic stimuli and disruptions in the functioning of the central auditory system can affect the development of hearing. UHL in children during their early years may negatively impact their verbal, linguistic, and communicative development[22]. Animal studies showed that even mild to moderate artificially induced unilateral conductive HL led to monaural deprivation in rats. Consequently, tonotopic maps were distorted, the deprived ear's respresentation was weakened. Limited auditory input due to UHL led to maladaptive plasticity throughout the critical stages of auditory cortex development and reorganization[23].These findings indicate that children with UHL, regardless of type and degree, can access speech and language with their normally hearing ear. Consistent with these hypotheses, our findings indicate that in terms of receptive and expressive language abilities, children with UHL caused by AA and SSD lagged behind their NH peers, and that there is no difference in the language development of children with UAA and SSD.
Furthermore, we included children with UAA and SSD who did not use any amplification devices, thus they achieved speech and language development with their normally hearing ear. We assumed that the lack of difference in receptive and expressive language skills between the two groups could be related to these factors. It is well-established that early intervention and the selection of an appropriate amplification method are crucial for a child's language and speech development, relevant to both bilateral and unilateral hearing loss[22].
The amplification methods for children with UHL could include behind-the-ear hearing aids, bone-anchored hearing aids, contralateral routing of signal systems, frequency modulation systems, and cochlear implants, dependent on various variables such as the degree and type of HL, chronological age, age at identification, and the anatomical function of the auditory system[2,13]. Therefore, it is recommended for future research to investigate the impact of amplification systems on language skills in children with different degree and types of UHL. In addition to amplification use, parent-child interaction is very important for children's language development. Because, parents are seen as the main provider of the linguistic stimulation required for child language development. Each parent and child is different and clinical time spent assessing each individual's characteristics is important for target tailoring and implementing intervention succesfully[24]. This intervention also include the educational support. Research indicates that children with UHL need tailored education and rehabilitation strategies that support their speech and language development[25]. Future research should evaluate the language development of children with UHL, considering parent-child interactions and special education support.
In addition to the recommendations, there were several limitations and weaknesses in this study. Firstly, we evaluated language development in this study exclusively in terms of receptive and expressive language. Standardized measures are required for a more comprehensive language examination that assesses linguistic abilities, including morphosyntactic and grammatical components. This constitutes one of the study's limitations. The second limitation of this study is that the number of participants is limited, despite the fact that it includes a wide age range. This was important in assessing the impact of different types of unilateral hearing loss on language development during childhood. However , the number of participants was limited due to attempts to create homogeneous groups and the exclusion of individuals with syndromic hearing loss and additional disabilities with UHL. It is recommended to conduct similar studies with a higher number of participants in future studies.Thirdly, the effects of UHL were only assessed cross-sectionally, and the longitudinal effects of UHL on the same groups remain unknown. By longitudinally assessing the language abilities of participants, we could potentially obtain significant insights into the progressive consequences of hearing loss on language proficiency. Fourthly, five of the pariticipants in the SSD group had CN aplasia, CN aplasia could have a greater negative impact on language development. Therefore, children with SSD due to CN aplasia could be evaluated as a separate group in future investigations. Fifthly, the study included individuals who were referred to our department for auditory rehabilitation evaluation after undergoing hearing tests. In the group of individuals with UAA, the air conduction hearing thresholds of the atretic ear were not evaluated due to the difficulty of the young age group to cooperate with behavioral evaluations. As a result, the degree of hearing loss remains unknown in children with UAA. Lastly, in order to compare the effects of different types of UHL on language skills in more depth, further studies should include individuals with different types of UHL in addition to UAA and SSD.
The targeting of a homogeneous population of children with UHL in terms of age, gender, HL side (right/left), non-syndromic HL, and maternal education one of the strengths of this study. Even though the sample size was small, we believed that the inclusion of the homogeneous group in terms of the aforementioned characteristics strengthened the study. A second strength is the including of children with NH, which we believe to be more representative than based on test norms. Considering all of this, we believe that this study will contribute to the body of knowledge regarding the impact of various degrees and types of UHL on language ability.
Conclusion
The lack of differences between the SSD and UHL groups and the poorer language skills compared to those with NH suggest that children with UAA and SSD appear to have significant risk for receptive and expressive language delays. In order to reduce the possibility that these children will lag behind their peers in receptive and expressive language, it is crucial that their language development should be evaluated carefully. Also, further studies are needed to determine what kinds of auditory amplification or special education are effective in rehabilitating children with UHL for their delayed language development.
Conflict of Interest
No potential conflict of interest was reported by the authors.
Funding
No funding
Reference
1) Pittman AL, de Diego-Lázaro B. What can a child do with one normal-hearing ear? Speech perception and word learning in children with unilateral and bilateral hearing losses relative to peers with normal hearing. Ear and hearing. 2021;42(5):1228-37. [ Özet ]
2) Lieu JE. Management of children with unilateral hearing loss. Otolaryngologic Clinics of North America. 2015;48(6):1011-26. [ Özet ]
3) Kay-Rivest E, Irace AL, Golub JS, Svirsky MA. Prevalence of single-sided deafness in the United States. The Laryngoscope. 2022;132(8):1652-6. [ Özet ]
4) Florentine MM, Le Clec'h S, Upton SM, Scarpelli C, Carr JP, Chan DK. Disparities in speech and language delay among children with Aural Atresia. Ear and Hearing. 2022;43(5):1574-81. [ Özet ]
5) Takeyama T, Shimada A, Sakamoto Y, Aoki T, Kondo E, Nakano S, Fukuda J, Azuma T, Sato G, Okamoto H, Kitamura Y, Udaka J, Takeda N. Development of receptive vocabulary and verbal intelligence in Japanese children with unilateral hearing loss. Auris Nasus Larynx. 2022;49(3):335-41. [ Özet ]
6) Yang F, Zheng Y, Li G. Early prelingual auditory development of infants and toddlers with unilateral hearing loss. Otology & Neurotology. 2020;41(5):654-00. [ Özet ]
7) Clark JG. Uses and abuses of hearing loss classification. Asha. 1981;23(7):493-500. [ Özet ]
8) Hresko WP, Reid DK, Hammill DD. TELD-3: Test of early language development: Pro-ed; 1999.
9) Topbaş S, Güven S. TEDİL: Türkçe Erken Dil Gelişim Testi Kullanım Klavuzu. TC: Detay Yayıncılık. 2013.
10) Cozad RL. Speechreading skill and communication difficulty of children and young adults with unilateral hearing loss. Journal of Auditory Research. 1977. [ Özet ]
11) Klee TM, Davis-Dansky E. A comparison of unilaterally hearing-impaired children and normal-hearing children on a battery of standardized language tests. Ear and Hearing. 1986;7(1):27-37. [ Özet ]
12) Anne S, Lieu JE, Cohen MS. Speech and language consequences of unilateral hearing loss: a systematic review. Otolaryngology-Head and Neck Surgery. 2017;157(4):572-9. [ Özet ]
13) Fitzpatrick EM, Gaboury I, Durieux-Smith A, Coyle D, Whittingham J, Nassrallah F. Auditory and language outcomes in children with unilateral hearing loss. Hearing research. 2019;372:42-51. [ Özet ]
14) Sangen A, Royackers L, Desloovere C, Wouters J, van Wieringen A. Single-sided deafness affects language and auditory development-a case-control study. Clinical Otolaryngology. 2017;42(5):979-87. [ Özet ]
15) Lieu JE, Tye-Murray N, Karzon RK, Piccirillo JF. Unilateral hearing loss is associated with worse speech-language scores in children. Pediatrics. 2010;125(6):e1348-e55. [ Özet ]
16) Lieu JE, Tye-Murray N, Fu Q. Longitudinal study of children with unilateral hearing loss. The Laryngoscope. 2012;122(9):2088-95. [ Özet ]
17) Montino S, Agostinelli A, Trevisi P, Martini A, Ghiselli S. Check-list for the assessment of functional impairment in children with congenital aural atresia. International Journal of Pediatric Otorhinolaryngology. 2017;102:174-9. [ Özet ]
18) Jensen DR, Grames LM, Lieu JE. Effects of aural atresia on speech development and learning: retrospective analysis from a multidisciplinary craniofacial clinic. JAMA Otolaryngology-Head & Neck Surgery. 2013;139(8):797-802. [ Özet ]
19) van Wieringen A, Boudewyns A, Sangen A, Wouters J, Desloovere C. Unilateral congenital hearing loss in children: Challenges and potentials. Hearing research. 2019;372:29-41. [ Özet ]
20) Tomblin JB, Harrison M, Ambrose SE, Walker EA, Oleson JJ, Moeller MP. Language outcomes in young children with mild to severe hearing loss. Ear and hearing. 2015;36(0 1):76S. [ Özet ]
21) Fitzpatrick EM, Crawford L, Ni A, Durieux-Smith A. A descriptive analysis of language and speech skills in 4-to 5-yr-old children with hearing loss. Ear and hearing. 2011;32(5):605-16. [ Özet ]
22) Rohlfs A-K, Friedhoff J, Bohnert A, Breitfuss A, Hess M, Müller F, Strauch A, Röhrs M, Wiesner T. Unilateral hearing loss in children: a retrospective study and a review of the current literature. European journal of pediatrics. 2017;176:475-86. [ Özet ]
23) Kaplan-Neeman R, Greenbom T, Habiballah S, Henkin Y. Biomarkers of auditory cortical plasticity and development of binaural pathways in children with unilateral hearing loss using a hearing aid. Hearing research. 2024; 451. [ Özet ]
24) Curtin M, Dirks E, Cruice M, Herman R, Newman L, Rodgers L, Morgan G. Assessing parent behaviours in parent-child ınteractions witih deaf and hard of hearing ınfants aged 0-3 years: a systematic review. Journal of clinical medicine. 2021;3345. [ Özet ]
25) Smit AL, Burgers YRW, Swanenburg de Veye HFN, Stegeman L, Breugem CC. Hearing-related quality of life, developmental outcomes and performance in children and young adults with unilateral conductive hearing loss due to aural atresia. 2021; 142. [ Özet ]